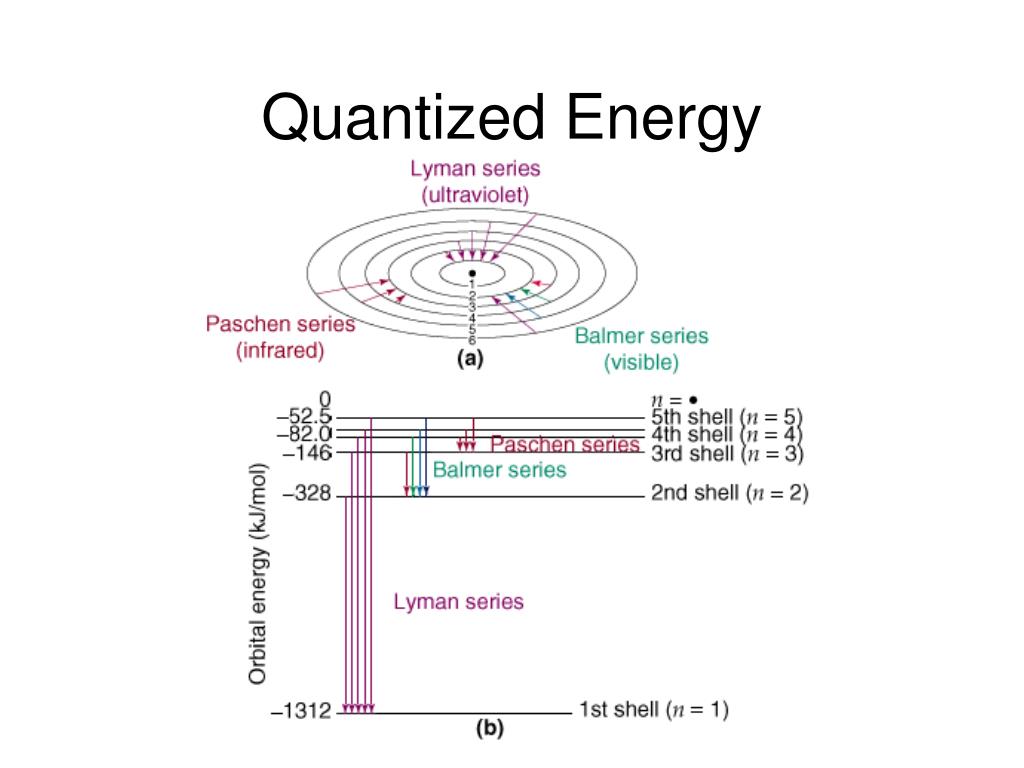
What Are Quantized Energy Levels . quantization of energy means that some systems can have only certain energies, not a continuous range. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and diagrams. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). the motion of the electron in the hydrogen atom is not free. quantization of energy means that some systems can have only certain energies, not a continuum of energies. learn how matter waves and particle energy levels are quantized in quantum mechanics. Spectroscopists often talk about energy and frequency as equivalent. The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. Learn how planck's constant, blackbody. learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation.
from www.slideserve.com
Learn how planck's constant, blackbody. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and diagrams. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). the motion of the electron in the hydrogen atom is not free. The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. quantization of energy means that some systems can have only certain energies, not a continuum of energies. quantization of energy means that some systems can have only certain energies, not a continuous range. learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. learn how matter waves and particle energy levels are quantized in quantum mechanics. Spectroscopists often talk about energy and frequency as equivalent.
PPT The Quantum Mechanical Model of the Atom PowerPoint Presentation
What Are Quantized Energy Levels quantization of energy means that some systems can have only certain energies, not a continuum of energies. The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. quantization of energy means that some systems can have only certain energies, not a continuous range. quantization of energy means that some systems can have only certain energies, not a continuum of energies. the motion of the electron in the hydrogen atom is not free. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). learn how matter waves and particle energy levels are quantized in quantum mechanics. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and diagrams. Spectroscopists often talk about energy and frequency as equivalent. learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. Learn how planck's constant, blackbody.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation ID2061071 What Are Quantized Energy Levels Spectroscopists often talk about energy and frequency as equivalent. quantization of energy means that some systems can have only certain energies, not a continuum of energies. learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. learn how matter waves and particle energy levels are quantized in quantum mechanics.. What Are Quantized Energy Levels.
From kdi-ppi.com
Exploring Energy Level Diagram Examples From Atoms to Molecules What Are Quantized Energy Levels The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and diagrams. learn how matter waves and particle energy levels are quantized in quantum mechanics. the motion of the electron in the hydrogen atom. What Are Quantized Energy Levels.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID5499863 What Are Quantized Energy Levels wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. Learn how planck's constant, blackbody. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and. What Are Quantized Energy Levels.
From www.researchgate.net
Calculated electron and hole quantized energy levels and wave functions What Are Quantized Energy Levels learn how matter waves and particle energy levels are quantized in quantum mechanics. Spectroscopists often talk about energy and frequency as equivalent. quantization of energy means that some systems can have only certain energies, not a continuous range. quantization of energy means that some systems can have only certain energies, not a continuum of energies. Learn how. What Are Quantized Energy Levels.
From www.slideserve.com
PPT The Quantum Mechanical Model of the Atom PowerPoint Presentation What Are Quantized Energy Levels Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and diagrams. Spectroscopists often talk about energy and frequency as equivalent. learn how matter waves and particle energy levels are quantized in quantum mechanics. the motion of the electron in the hydrogen atom is not free. quantization of energy means. What Are Quantized Energy Levels.
From www.slideserve.com
PPT Energy is Quantized PowerPoint Presentation, free download ID What Are Quantized Energy Levels quantization of energy means that some systems can have only certain energies, not a continuum of energies. learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. learn how matter waves and particle energy levels are quantized in quantum mechanics. The electron is bound to the atom by the. What Are Quantized Energy Levels.
From www.slideserve.com
PPT Modern Physics PowerPoint Presentation, free download ID6579365 What Are Quantized Energy Levels wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. learn how matter waves and particle energy levels are quantized in quantum mechanics. the motion of the electron in. What Are Quantized Energy Levels.
From pressbooks.bccampus.ca
8.2 Quantization of the Energy of Electrons CHEM 1114 Introduction What Are Quantized Energy Levels Spectroscopists often talk about energy and frequency as equivalent. quantization of energy means that some systems can have only certain energies, not a continuum of energies. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). Learn how planck's constant, blackbody. the motion of the electron in the. What Are Quantized Energy Levels.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID5499863 What Are Quantized Energy Levels learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. quantization of energy means that some systems can have only certain energies, not a continuum of energies. Learn how planck's constant, blackbody. learn how matter waves and particle energy levels are quantized in quantum mechanics. the motion of. What Are Quantized Energy Levels.
From www.slideserve.com
PPT The Quantum Mechanical Atom CHAPTER 7 Chemistry The Molecular What Are Quantized Energy Levels learn how matter waves and particle energy levels are quantized in quantum mechanics. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and diagrams. quantization of energy means that some systems can have only certain energies, not a continuous range. Spectroscopists often talk about energy and frequency as equivalent. The. What Are Quantized Energy Levels.
From naomiwade.z21.web.core.windows.net
Electrons In Energy Levels Chart What Are Quantized Energy Levels The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). Spectroscopists often talk about energy and frequency as equivalent. Learn how planck's constant, blackbody. learn how to describe the energy levels of. What Are Quantized Energy Levels.
From slideplayer.com
Evolution of the Atomic Model ppt download What Are Quantized Energy Levels learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. Spectroscopists often talk about energy and frequency as equivalent. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). Learn how planck's constant, blackbody. the motion of the electron in. What Are Quantized Energy Levels.
From www.slideserve.com
PPT Modern Atomic Theory PowerPoint Presentation, free download ID What Are Quantized Energy Levels quantization of energy means that some systems can have only certain energies, not a continuous range. Learn how planck's constant, blackbody. quantization of energy means that some systems can have only certain energies, not a continuum of energies. learn how matter waves and particle energy levels are quantized in quantum mechanics. learn how to describe the. What Are Quantized Energy Levels.
From www.slideserve.com
PPT Radiation An Introduction to Light and Quantized What Are Quantized Energy Levels Learn how planck's constant, blackbody. learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. quantization of energy means that some systems can have only certain energies, not a continuous range. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u. What Are Quantized Energy Levels.
From physics.stackexchange.com
quantum mechanics Quantized energy levels of Bohr hydrogen atom What Are Quantized Energy Levels Learn how planck's constant, blackbody. The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. learn how matter waves and particle energy levels are quantized in quantum mechanics. Spectroscopists often talk about energy and frequency as equivalent. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions. What Are Quantized Energy Levels.
From shortcircuitweb.com
Understanding Energy Level Diagrams in Chemistry A Comprehensive Guide What Are Quantized Energy Levels wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u \). The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. Explore the concepts of de broglie wavelength, matter standing waves, energy spectrum, and energy transitions with examples and diagrams. learn how matter. What Are Quantized Energy Levels.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube What Are Quantized Energy Levels quantization of energy means that some systems can have only certain energies, not a continuum of energies. learn how matter waves and particle energy levels are quantized in quantum mechanics. Learn how planck's constant, blackbody. The electron is bound to the atom by the attractive force of the nucleus and consequently quantum. wavelength is inversely proportional to. What Are Quantized Energy Levels.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry What Are Quantized Energy Levels the motion of the electron in the hydrogen atom is not free. learn how to describe the energy levels of electrons in atoms using quantum mechanics and the rydberg equation. Spectroscopists often talk about energy and frequency as equivalent. wavelength is inversely proportional to energy but frequency is directly proportional as shown by planck's formula, e=h\( u. What Are Quantized Energy Levels.